Medtronic’s Hugo RAS System Hits Trial Milestone
In a significant development in the field of robotic-assisted urologic surgery, Medtronic’s Hugo RAS system has achieved a major milestone in its U.S. clinical trial. The Expand URO study, the largest trial of its kind in the United States, has shown that the Hugo RAS system meets both safety and effectiveness goals. With a remarkable 98.5% surgical success rate and low complication rates, the study provides robust support for Medtronic’s FDA submission for urologic use.
The findings of the Expand URO study were presented at the American Urological Association (AUA) annual meeting in Las Vegas, providing a significant update on the clinical performance of the Hugo RAS system. The system is designed to assist surgeons in performing complex urologic procedures with enhanced precision, dexterity, and visualization.
The Expand URO study is a prospective, randomized controlled trial that enrolled 250 patients at 20 sites across the United States. The study aimed to evaluate the safety and effectiveness of the Hugo RAS system for the treatment of urologic procedures, including partial nephrectomy and prostatectomy. The primary endpoints of the study included surgical success rate, complication rate, and patient-reported outcomes.
The results of the study are impressive, with a 98.5% surgical success rate and a low complication rate of 10.8%. The study also showed that the Hugo RAS system is associated with improved patient-reported outcomes, including reduced pain, improved quality of life, and enhanced patient satisfaction.
The success of the Expand URO study is a significant milestone for Medtronic, as it paves the way for the company’s FDA submission for urologic use. The study provides robust clinical evidence supporting the safety and effectiveness of the Hugo RAS system for urologic procedures, and it is expected to play a key role in the company’s regulatory submission.
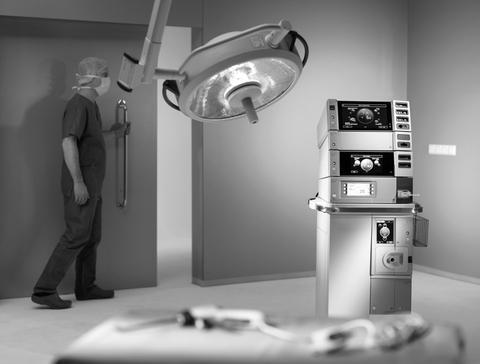
The Hugo RAS system is a state-of-the-art robotic-assisted surgery (RAS) platform that is designed to provide surgeons with advanced visualization, precision, and control during complex urologic procedures. The system features a high-definition 3D visualization system, advanced instrumentation, and ergonomic design to enhance the surgical experience.
The system is also compatible with a range of instruments and accessories, allowing surgeons to perform a variety of procedures, including partial nephrectomy, prostatectomy, and cystectomy. The system is also designed to provide real-time feedback and data to surgeons, allowing them to make more informed decisions during the procedure.
The success of the Expand URO study is a testament to the potential of the Hugo RAS system to improve patient outcomes and reduce complications in urologic surgery. The study provides a strong foundation for further research and development of the system, and it is expected to have a significant impact on the field of urologic surgery.
In conclusion, the Expand URO study is a major milestone in the development of the Hugo RAS system, and it provides robust clinical evidence supporting the safety and effectiveness of the system for urologic procedures. The study’s findings are expected to play a key role in Medtronic’s FDA submission for urologic use, and they will likely have a significant impact on the field of urologic surgery.