Neuralink Rival Precision Gets FDA Nod for Brain Implant Component
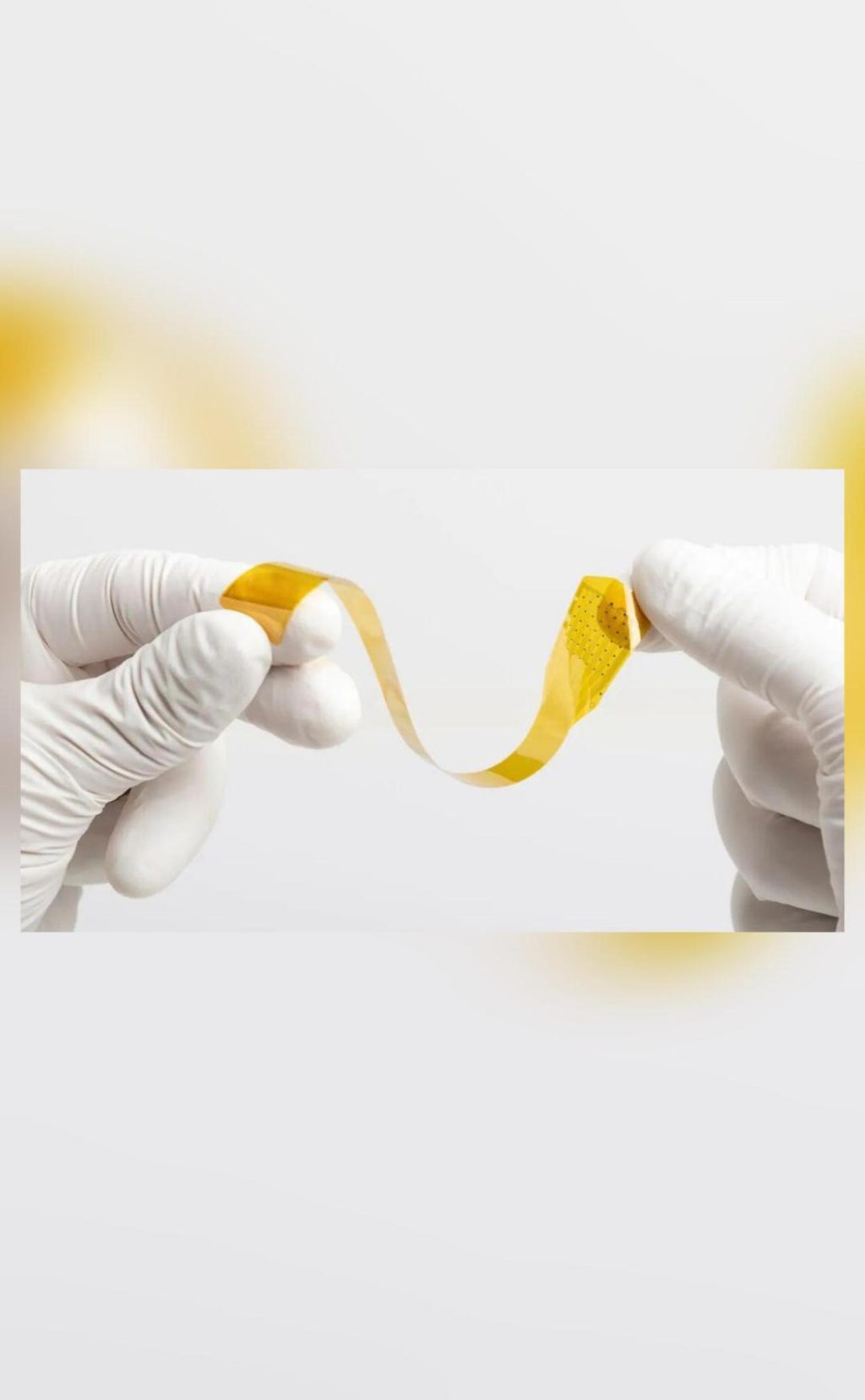
In a significant breakthrough, Precision Neuroscience, a company that is often considered a rival to Elon Musk’s Neuralink, has received approval from the US Food and Drug Administration (FDA) for its brain implant system’s critical component, the Layer 7 Cortical Interface. This high-resolution cortical electrode array is designed for recording, monitoring, and stimulation of electrical activity on the surface of the brain. The clearance allows the array to remain implanted for up to 30 days.
The FDA approval is a major milestone for Precision Neuroscience, which is focused on developing innovative solutions for paralyzed patients. The company’s technology is designed to enable individuals with paralysis to control devices using only their thoughts. The Layer 7 Cortical Interface is a crucial part of this technology, allowing for the precise recording and stimulation of neural signals.
The FDA clearance is based on the results of a clinical trial conducted by Precision Neuroscience, which demonstrated the safety and efficacy of the Layer 7 Cortical Interface. The trial involved implanting the device in 10 patients with paralysis and assessing its ability to record and stimulate neural signals. The results showed that the device was able to accurately record neural activity and stimulate muscle contractions, allowing patients to control devices such as prosthetic limbs.
“This is a significant milestone for Precision Neuroscience and for the development of brain-computer interfaces (BCIs) in general,” said Dr. Tim McMurtrey, Chief Medical Officer at Precision Neuroscience. “Our technology has the potential to revolutionize the treatment of paralysis and other neurological disorders by enabling individuals to control devices using only their thoughts.”
Precision Neuroscience’s technology is based on a novel approach to BCI development, which involves using a high-resolution cortical electrode array to record and stimulate neural signals. The device is designed to be implantable and can remain in place for extended periods of time, allowing for continuous monitoring and stimulation of neural activity.
The FDA approval is a major coup for Precision Neuroscience, which is often compared to Neuralink, a company founded by Elon Musk that is also developing BCI technology. While Neuralink has received significant attention and funding, Precision Neuroscience has been quietly working on its own BCI technology and has made significant progress in recent years.
“This is a significant win for Precision Neuroscience and for the development of BCI technology,” said Dr. McMurtrey. “We are thrilled to have received FDA clearance for our Layer 7 Cortical Interface and look forward to continuing to develop our technology to help paralyzed patients regain control over their lives.”
The FDA approval is just the latest development in Precision Neuroscience’s journey to develop BCI technology. The company has been working on its technology for several years and has made significant progress in recent years. In addition to the Layer 7 Cortical Interface, Precision Neuroscience is also developing a range of other BCI-related technologies, including brain-computer interface systems and neural prosthetics.
Overall, the FDA approval of Precision Neuroscience’s Layer 7 Cortical Interface is a major milestone for the company and for the development of BCI technology. The device has the potential to revolutionize the treatment of paralysis and other neurological disorders by enabling individuals to control devices using only their thoughts. As the technology continues to evolve, it is likely to have a significant impact on the lives of individuals with paralysis and other disabilities.
Source: